CRISPR gene editing is revolutionizing the landscape of genetic research and medical treatment, offering unprecedented precision in altering the DNA of living organisms. This groundbreaking gene editing technology has the potential to cure genetic diseases like sickle cell anemia, providing hope for millions. However, its rapid advancement raises significant ethical implications of CRISPR, as society grapples with questions about who should be allowed to edit the human genome and the long-term effects of such interventions. As we explore the risks and benefits of CRISPR, critical discussions around health justice and gene editing emerge, emphasizing the need for equitable access to these innovations. The conversation surrounding CRISPR is not just about science; it encompasses our values and priorities as a society in the face of transformative genetic capabilities.
The emergence of gene modification techniques, particularly through CRISPR technology, presents a pivotal moment in scientific advancement. This powerful tool allows researchers to implement precise genetic alterations, paving the way for potential treatments of hereditary conditions such as sickle cell disease. Yet, as these gene-altering methods gain traction, they also usher in pressing ethical debates about our responsibilities toward these advancements. The discourse extends beyond mere medical breakthroughs to considerations of fairness in healthcare access and the societal ramifications of gene editing. As we navigate the complex terrain of genetic interventions, the discussions about ethical implications, health equity, and the societal impact of gene editing grow increasingly essential.
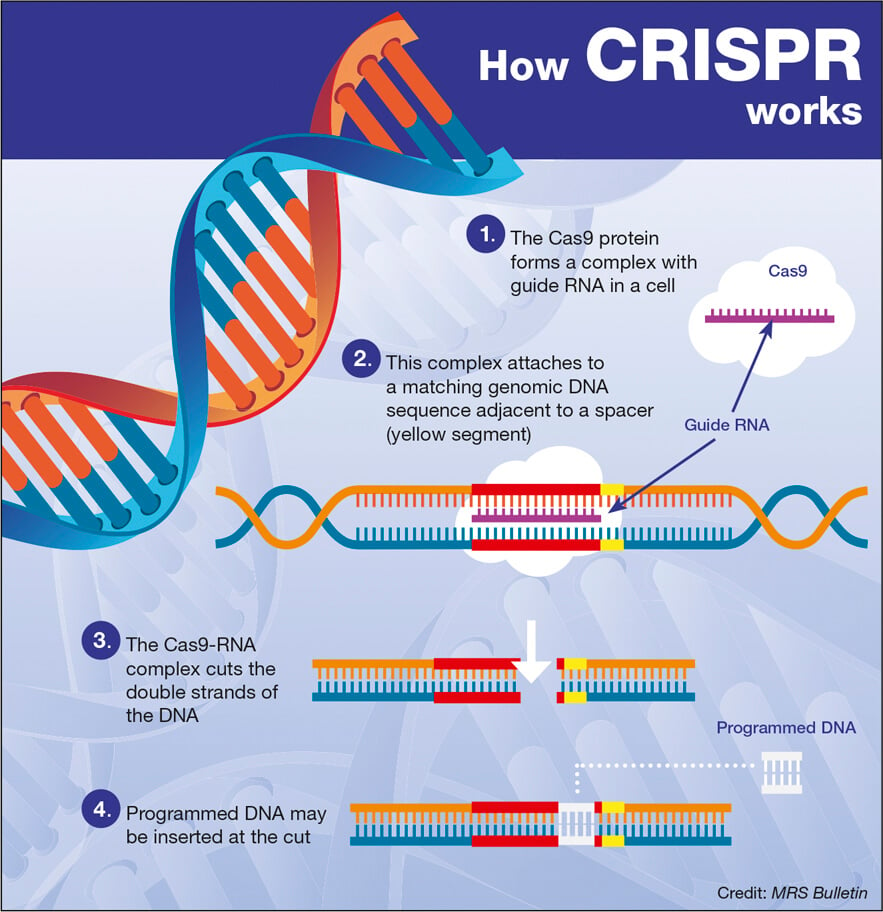
Understanding CRISPR Gene Editing Technology
CRISPR gene editing is a revolutionary technology that offers the ability to precisely manipulate DNA sequences within living organisms. This method, derived from a natural defense mechanism of bacteria, allows scientists to target specific genes for modification, effectively ‘cutting out’ unwanted traits or diseases. By utilizing guide RNA to direct an enzyme, known as Cas9, to the precise location in the genome, CRISPR enables genetic changes that have the potential to cure genetic disorders such as sickle cell anemia, hereditary blindness, and even certain cancers.
The versatility of CRISPR offers both somatic and germline gene editing capabilities. Somatic gene editing affects only specific tissues and does not pass on changes to offspring, whereas germline editing involves modifying eggs or sperm, making changes heritable. While the promise of eradicating inherited disorders is profound, the technology has sparked intense debate about the ethical implications of germline editing. As we stand on the brink of genomic advancements, it is critical to consider not only the scientific possibilities but also the societal responsibilities that come with them.
Ethical Implications of CRISPR Gene Editing
The introduction of CRISPR gene editing technology raises a host of ethical concerns that society must grapple with as we advance scientifically. One of the foremost questions is whether it is acceptable to alter human genes, particularly when the changes could affect future generations. For instance, if we have the means to eliminate conditions such as sickle cell anemia, should we also consider modifying genes associated with traits like intelligence or physical ability? This touches on the moral dilemma of ‘playing God’ and the potential consequences of creating ‘designer babies’, leading to calls for robust ethical guidelines.
Moreover, the financial ramifications of CRISPR technology add another layer to the ethical discourse. Given that the cost of gene therapies can reach millions, accessibility becomes a pressing issue. Health justice concerns arise as these treatments may not be equitably distributed, favoring those with the means to afford them, while leaving vulnerable populations at risk of exclusion. Experts like Neal Baer emphasize the necessity of incorporating ethical considerations, particularly regarding who has access to such groundbreaking treatments and who has the authority to make decisions about gene editing.
CRISPR and Sickle Cell Anemia Treatment
Sickle cell anemia presents a significant challenge in medicine, where patients often endure severe complications, including strokes and organ damage. With the advent of CRISPR gene editing technology, there is newfound hope for effective treatments that could transform the lives of those afflicted. By editing the genes responsible for producing defective hemoglobin, CRISPR holds the potential to not just alleviate symptoms but offer a permanent cure for sickle cell anemia, making it a focal point of current biotechnological research.
While the excitement surrounding CRISPR offers a glimpse of potential cures, it also raises critical concerns regarding safety and the long-term implications of such genetic modifications. The need for rigorous clinical trials and ethical oversight cannot be overstated, especially in light of the cost associated with CRISPR therapies. With treatments priced at around $2.2 million each, it’s imperative to address the questions of who will benefit from these advancements and how society will respond to the resulting disparities in health equity.
Health Justice and Gene Editing
Health justice is a vital concept in the discourse surrounding CRISPR gene editing and medical innovation. As new technologies become available, there is a worry that they may exacerbate existing health disparities. When discussing CRISPR and its applications, one must consider how the wealthy will likely have greater access to cutting-edge treatments, while marginalized communities may continue to suffer from neglected diseases. The challenge lies in ensuring that advancements in gene editing technology are coupled with a commitment to equity that prioritizes the health of all populations.
Experts argue that the introduction of CRISPR requires not only scientific regulation but also a framework that fosters health equity. Creating policies that dictate the responsible use of gene editing technologies involves collaboration among policymakers, healthcare providers, and communities. This conversation is crucial in preventing a future where access to genetic cures becomes another privilege of socioeconomic status rather than a fundamental right for all individuals.
CRISPR: Weighing Risks and Benefits
The utilization of CRISPR gene editing technology comes with a complex interplay of risks and benefits that must be carefully assessed. While the ability to eradicate genetic diseases presents tremendous potential, it also fuels concerns regarding unintended consequences. Modifying genes can lead not only to the desired outcomes but could inadvertently introduce new health issues or ecological disruptions. For instance, alterations to genes might interact in unforeseen ways, highlighting the need for comprehensive research and ethical surveillance before widespread application.
Moreover, the prospect of enhancing human traits raises ethical questions about who gets to define the ‘ideal’ human being. Critics worry about the implications of selecting for specific characteristics, which could lead to a societal divide where certain traits are viewed as superior. This introduces moral complexity to the discussion, underscoring the need for an inclusive dialogue that encompasses various perspectives on what constitutes a beneficial application of CRISPR technology in society.
The Future of Gene Editing Legislation
As CRISPR gene editing technology grows in capability, so too does the necessity for comprehensive legislation to manage its use. Current laws surrounding genetic editing are often outdated and do not adequately address the rapid pace of advancements in biotechnology. Legislators are faced with the challenging task of crafting regulations that protect against misuse while fostering innovation and public trust. As the ramifications of gene editing become increasingly significant, the push for robust legislative frameworks is becoming more urgent.
Future legislation must consider ethical frameworks that dictate not only the safety and efficacy of CRISPR interventions but also their accessibility and social implications. Discussions around these laws should involve a wide range of stakeholders, including ethicists, scientists, healthcare providers, and community members. By creating laws that foresee the intricate challenges posed by gene editing, we can ensure that CRISPR technologies are used responsibly, promoting the common good while minimizing risks.
Public Perception of Gene Editing Technology
The public perception of CRISPR and gene editing technology is pivotal in shaping its future acceptance and regulation. As awareness grows about the potential of gene editing to cure genetic diseases, it is vital to also communicate the complexities and ethical dilemmas that accompany these advancements. Misinformation or lack of understanding can lead to fear and resistance, which may hinder scientific progress. Therefore, effective education and clear communication strategies are essential for bridging the gap between scientific communities and the general public.
Furthermore, public engagement in discussions about gene editing can lead to more informed opinions and societal consensus on where these technologies should be headed. As individuals become more proactive in voicing their concerns and hopes, it fosters a culture of accountability among researchers and policymakers. Engaging communities in these discussions ensures that advancements are aligned with societal values and ethical standards, ultimately steering CRISPR technology towards the common good.
CRISPR’s Role in Treating Genetic Disorders
CRISPR gene editing stands at the forefront of innovative approaches for treating genetic disorders, revolutionizing traditional methods of medical intervention. The technology offers unparalleled precision and efficiency, making it possible to target specific mutations responsible for conditions such as cystic fibrosis, Huntington’s disease, and muscular dystrophy. By correcting these genetic defects at their source, CRISPR holds the promise of not just managing symptoms, but potentially curing previously untreatable diseases, ushering in a new era of genetic medicine.
However, the intricacies of gene editing extend beyond technical capabilities; they weave into the fabric of patient care and societal expectations. As research progresses towards clinical applications of CRISPR, it is imperative to remain vigilant regarding the ethical considerations and patient autonomy. The dialogue surrounding informed consent, potential long-term effects, and access to therapies is crucial to ensuring that the benefits of gene editing are harnessed responsibly for today’s patients and future generations.
The Ethics of Gene Modification Decision-Making
The moral quandaries associated with gene modification extend to the decision-making processes involved in utilizing CRISPR technology. Questions emerge about who should hold the power to decide how and when CRISPR should be applied, particularly concerning germline editing, which can produce heritable changes. Parents contemplating genetic modifications for their children may face intense scrutiny, highlighting the necessity for regulations and ethical guidelines that govern these choices. Engaging diverse communities in these discussions is vital to capture a broad spectrum of values and beliefs.
Understanding these ethical implications fosters accountability among developers and researchers, ensuring that gene editing is approached with caution. The necessity of establishing an ethics board to oversee CRISPR applications could provide a framework for ethical decision-making characterized by transparency and public involvement. This collaborative approach may empower communities to come together to navigate the complex challenges and choices posed by gene editing technology.
Frequently Asked Questions
What are the ethical implications of CRISPR gene editing?
The ethical implications of CRISPR gene editing are significant and multifaceted. They include concerns about consent, particularly when editing germline cells that affect future generations. There are also debates surrounding ‘designer babies’ and the right to alter human traits. The conversation is further complicated by the disparities in access to CRISPR technology, raising questions about health justice and potential inequalities based on socioeconomic status.
How is CRISPR being used to treat sickle cell anemia?
CRISPR gene editing technology is being utilized to treat sickle cell anemia by directly altering the genes associated with the disease. This approach involves editing somatic cells to remove the genetic mutations responsible for sickle cell, effectively curing the disease in individuals. The potential to edit germline cells adds another layer, as it could prevent the disease from manifesting in future generations.
What are the risks and benefits of CRISPR technology?
The benefits of CRISPR technology include the potential to cure genetic diseases like sickle cell anemia and improve agricultural resilience. However, the risks involve unintended genetic changes or off-target effects that could lead to other health issues. Additionally, ethical concerns arise regarding how and when to use CRISPR, particularly regarding traits and enhancements that may not be considered pathological.
What role does health justice play in gene editing technology?
Health justice is a crucial consideration in gene editing technology like CRISPR. As advancements in gene editing create potential cures for diseases, issues of access and equity become paramount. If only certain populations can afford treatments like CRISPR for sickle cell anemia, disparities in health outcomes will persist. The need for equitable access to these technologies is essential to ensure that health benefits are distributed fairly across all communities.
Can CRISPR be used responsibly in medical treatments?
CRISPR can be used responsibly in medical treatments if ethical guidelines, robust oversight, and comprehensive risk assessments are established. This involves careful consideration of which diseases to target and ensuring informed consent for treatments, particularly with germline editing. Ongoing discussions among ethicists, scientists, and policymakers are necessary to navigate the complexities of responsible CRISPR gene editing.
| Key Points | Details |
|---|---|
| Ethical Debate | Discussion on whether we should edit human genes, particularly in cases like sickle cell anemia. |
| CRISPR Overview | CRISPR allows for the editing of somatic and germline genes, presenting both opportunities and ethical dilemmas. |
| Cost and Accessibility | The cure for sickle cell anemia costs approximately $2.2 million, raising concerns about health equity. |
| Social Implications | The possibility of enhancing traits raises questions about parental rights and societal impacts. |
| Global Concerns | Uneven application and oversight of gene editing technology in different countries pose ethical risks. |
| Unintended Consequences | Genetic changes may have unforeseen effects due to the complex interactions of genes. |
Summary
CRISPR gene editing is a revolutionary technology that offers the potential to cure genetic diseases but raises significant ethical questions. The discussion surrounding CRISPR highlights the need to balance innovation with ethical considerations, particularly regarding the implications of altering human traits and the responsibility that comes with such power. As we navigate this landscape, it is crucial to address issues of accessibility, oversight, and the social ramifications of gene editing to ensure fair and just outcomes for all individuals.