Recent advances in TIM-3 therapy for Alzheimer’s shed new light on potential treatments for this devastating condition, linking the TIM-3 molecule to the brain’s immune response. Researchers have uncovered that TIM-3 acts as a checkpoint inhibitor, preventing microglia from effectively clearing amyloid plaques associated with Alzheimer’s disease. By targeting and inhibiting this molecule, scientists aim to unleash the brain’s immune cells, which could lead to cognitive improvement in mice and possibly human patients alike. This novel approach capitalizes on the immune system’s mechanisms, traditionally exploited in cancer immunotherapy, to tackle the urgent need for better Alzheimer’s disease treatments. As we delve deeper into how TIM-3 interacts within the neural landscape, exciting possibilities emerge for enhancing memory function and overall brain health.
Exploring innovative therapies to combat Alzheimer’s disease, TIM-3 therapy stands out as a promising strategy. The focus is on a key immune checkpoint, TIM-3, which regulates microglial activity in the brain, limiting their ability to eliminate harmful plaques. With recent research highlighting the connection between TIM-3 and cognitive functions, particularly in murine models, there’s optimism for advancing treatment options. As understanding of microglia and their role in neurodegenerative conditions grows, the potential to redefine Alzheimer’s treatments through immune modulation becomes more tangible. This groundbreaking work opens avenues for developing therapies that could restore cognitive abilities and improve the quality of life for those affected by Alzheimer’s.
Understanding TIM-3: A Breakthrough in Alzheimer’s Therapy
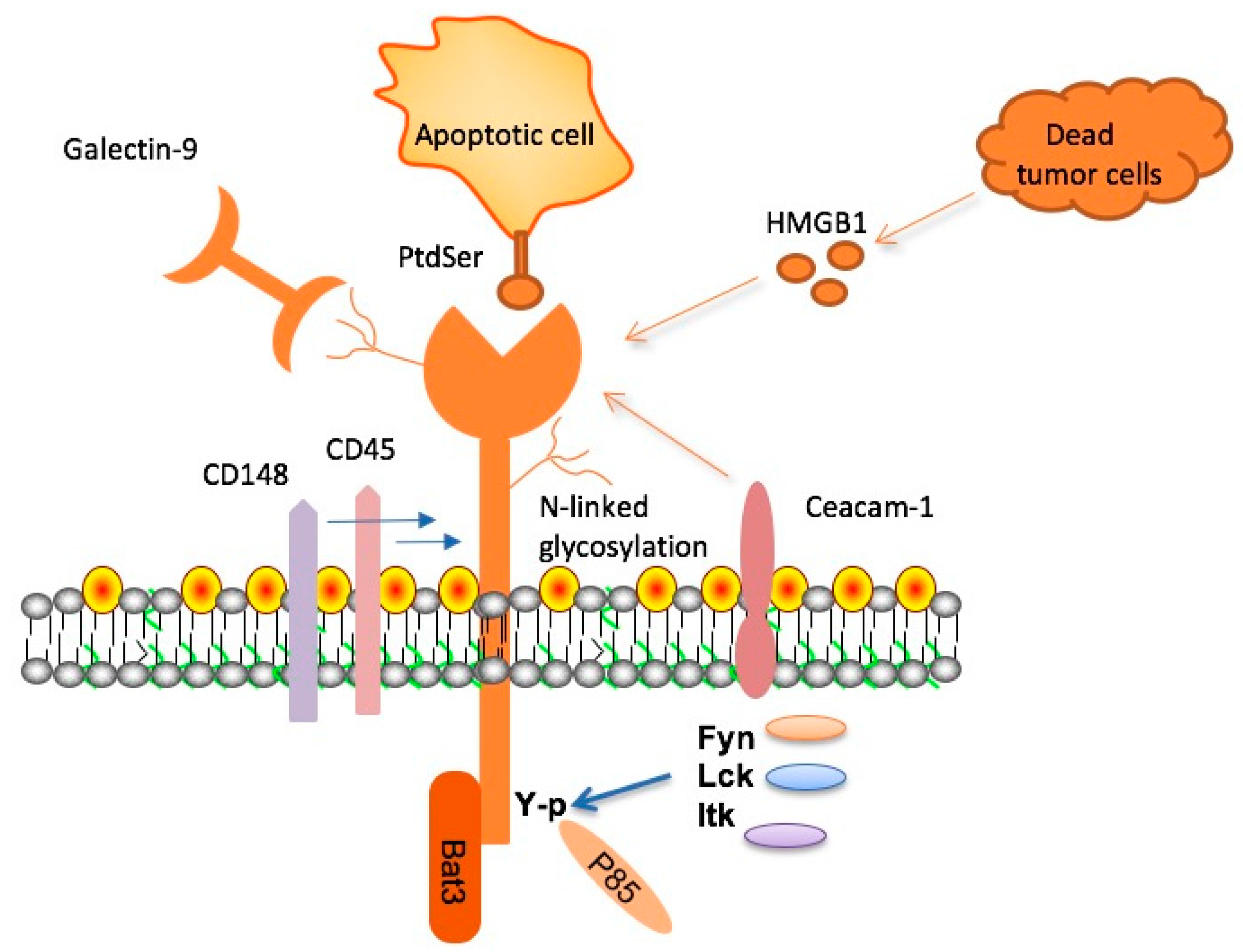
TIM-3, or T-cell immunoglobulin mucin-3, is a checkpoint molecule that plays a significant role in regulating immune responses. Recent studies have indicated that this molecule, which is often utilized in cancer therapies, could be pivotal in treating Alzheimer’s disease by modifying the actions of microglia. These immune cells in the brain typically clear harmful amyloid plaques, but the presence of TIM-3 inhibits their function, leading to plaque accumulation. Therefore, targeting TIM-3 presents a promising avenue to restore microglial activity and potentially enhance cognitive function in Alzheimer’s patients.
Research conducted on mice genetically modified to lack TIM-3 has revealed remarkable results, including significant reductions in plaque levels and cognitive improvements. By understanding the inhibitory mechanism of TIM-3, scientists are exploring the possibility of developing therapies that could inhibit this checkpoint molecule, allowing microglia to effectively combat Alzheimer’s pathology. Thus, TIM-3 therapy could revolutionize Alzheimer’s disease treatments by freeing up the brain’s immune response to clear plaques more efficiently.
The Role of Microglia in Alzheimer’s Disease
Microglia serve as the brain’s primary immune defenders, constantly monitoring the environment and responding to damage. In the context of Alzheimer’s disease, these cells fail to clear amyloid-beta plaques due to the overexpression of inhibitory molecules like TIM-3. This homeostatic control prevents microglia from acting effectively, exacerbating the degeneration associated with Alzheimer’s. As microglial cells become increasingly dysfunctional with age, the accumulation of amyloid plaques leads to cognitive decline, highlighting the need to restore the natural function of these vital cells.
Recent studies have emphasized the duality of microglial functions: while they are crucial for pruning unused synapses during brain development, their role shifts detrimental in the presence of Alzheimer’s pathology. By targeting TIM-3, researchers aim to enhance microglial activation and promote plaque removal, ultimately leading to cognitive improvement in affected individuals. Thus, understanding microglia and their interactions with TIM-3 is essential for developing effective Alzheimer’s disease treatments.
Innovative Cancer Therapies Applied to Alzheimer’s Research
Historically, immune checkpoint inhibitors have made waves in cancer treatment by unleashing T-cells against tumors, and now, the same principles are being applied to Alzheimer’s research. By inhibiting TIM-3, which restrains microglial activity, scientists hope to replicate the success seen in oncology within the realm of neurodegenerative diseases. This innovative cross-disciplinary approach opens up possibilities for novel therapies that could halt or even reverse neurodegeneration by empowering the brain’s immune response.
Transitioning strategies from cancer therapy to Alzheimer’s aims not only at managing symptoms but also at tackling the root cause of cognitive decline. Successful manipulation of TIM-3 expression in microglia could lead to restored memory function and a better quality of life for Alzheimer’s patients. As more research unfolds, the exploration of immune checkpoint inhibitors could pave the way for groundbreaking treatments that address underlying pathologies effectively.
The Genetic Link: TIM-3 and Alzheimer’s Disease
Research has identified TIM-3 as a genetic risk factor for late-onset Alzheimer’s disease, with a polymorphism in the HAVCR2 gene correlating with its expression levels. This connection suggests that individuals with higher TIM-3 expression on microglial cells are more susceptible to Alzheimer’s pathology. Understanding the genetic basis of TIM-3 could help identify populations at risk and guide the development of targeted therapies that adjust immune responses.
Furthermore, these genetic insights allow for better stratification in clinical trials when testing TIM-3 targeted therapies. Patients can be grouped based on their genetic profiles, enhancing the likelihood of successful treatment outcomes. As clinical trials continue to reveal the efficacy of inhibiting TIM-3, they may usher in a new era of personalized medicine for Alzheimer’s patients.
Cognitive Reinstatement: Results from TIM-3 Research in Mouse Models
In laboratory studies, the elimination of TIM-3 from microglia in mouse models exhibiting Alzheimer’s disease has led to significant cognitive improvements. These mice demonstrated enhanced memory capabilities, showcasing that targeting TIM-3 can rejuvenate microglial function and markedly alter the disease’s trajectory. Animal studies highlight the potential to not only reduce plaque load but also to restore cognitive behavior that reflects improved memory and learning.
Behavioral tests, such as maze navigation, indicate a stark contrast between normal mice and those burdened by amyloid plaques. This research validates the hypothesis that by blocking TIM-3, microglia can effectively target and eliminate neurotoxic plaques, leading to cognitive restoration. The implications of these findings underscore the relevance of TIM-3 therapy in reestablishing normal cognitive functions in Alzheimer’s patients.
Future Directions in TIM-3 Therapy
Looking forward, the development of TIM-3 therapy for Alzheimer’s disease may involve creating monoclonal antibodies or small molecules to inhibit its activity. Early-phase clinical trials are crucial in evaluating the safety and efficacy of these therapies in humans, especially considering the previous challenges faced in Alzheimer’s drug development. With encouraging results from preclinical models, potential human applications are becoming more viable.
As research transitions from animal studies to human trials, the collaboration between institutions such as Harvard Medical School and the National Institutes of Health marks an optimistic step towards tangible solutions for Alzheimer’s. Should anti-TIM-3 antibodies prove effective in clinical settings, it could redefine the treatment landscape for this devastating disease, potentially improving outcomes for millions suffering from Alzheimer’s.
Challenges and Considerations in Developing TIM-3 Therapies
While the prospects of TIM-3 therapy for Alzheimer’s disease are promising, numerous challenges remain. Developing biologics that interact effectively with the central nervous system presents unique hurdles, including ensuring blood-brain barrier permeability and minimizing potential side effects. Furthermore, extensive long-term studies will be required to assess the impact of TIM-3 inhibition on brain health and overall cognitive function.
Equally important is the identification of appropriate biomarkers to monitor the therapeutic effects during clinical trials. By linking TIM-3 levels to cognitive changes and plaque burden, researchers can better evaluate the efficacy of these treatments. As the scientific community navigates these complexities, the hope remains that TIM-3 therapy could revolutionize Alzheimer’s treatments in a way that brings tangible benefits to patients.
A Multidisciplinary Approach to Alzheimer’s Treatment
Addressing Alzheimer’s disease through TIM-3 therapy will require a multidisciplinary approach that combines immunology, neurology, and pharmacology. Given the complexity of Alzheimer’s pathology, leveraging insights from multiple fields can lead to more comprehensive treatment strategies. Collaboration among researchers, clinicians, and pharmaceutical companies will be essential to translate lab findings into clinical solutions.
Additionally, educating healthcare providers and the public about the mechanisms behind TIM-3 and its role in Alzheimer’s is crucial for fostering support for ongoing research. Engaging the community will ensure that advancements in TIM-3 therapies receive the attention and funding necessary to advance through the stages of development and eventually bring hope to those affected by Alzheimer’s.
The Legacy of TIM-3 Research in Neuroscience
The ongoing investigation into the TIM-3 molecule is poised to leave a significant legacy in neuroscience and Alzheimer’s research. By understanding how this immune checkpoint interacts with microglia and contributes to pathogenic processes, researchers hope to open new pathways for intervention in diseases characterized by neurodegeneration. The successful application of TIM-3 therapies could symbolize a paradigm shift in how we approach not only Alzheimer’s but also other neurodegenerative diseases.
Moreover, the innovations arising from TIM-3 research may inspire further exploration into other immune checkpoint molecules, potentially yielding new targets for therapeutic intervention. As the field continues to evolve, the lessons learned from TIM-3 studies will undoubtedly impact future research and guide the quest for effective solutions to the significant challenges posed by Alzheimer’s disease.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s involves targeting the TIM-3 molecule, an immune checkpoint inhibitor that hinders the brain’s immune cells, or microglia, from clearing amyloid plaques. By blocking TIM-3, the therapy aims to reactivate microglia, enabling them to attack and eliminate these harmful plaques, potentially leading to cognitive improvement in Alzheimer’s patients.
How does the TIM-3 molecule relate to Alzheimer’s disease treatments?
The TIM-3 molecule is implicated in Alzheimer’s disease treatments as it acts as an inhibitory signal for microglia, the brain’s immune cells. In Alzheimer’s, these cells overexpress TIM-3, preventing them from clearing amyloid-beta plaques. By inhibiting TIM-3, treatments can potentially enhance the ability of microglia to remove these plaques, which is critical for cognitive function.
What role do microglia play in Alzheimer’s disease and TIM-3 therapy?
Microglia are the brain’s immune cells responsible for clearing waste, including amyloid-beta plaques associated with Alzheimer’s disease. In TIM-3 therapy, targeting the TIM-3 checkpoint molecule allows these microglia to regain functionality, attacking plaques that contribute to cognitive decline, thus improving memory and cognitive capabilities.
What evidence supports the effectiveness of TIM-3 therapy for cognitive improvement in mice?
Research has demonstrated that genetically deleting the TIM-3 gene in mouse models of Alzheimer’s led to significant plaque clearance and cognitive improvements. Mice without TIM-3 showed enhanced memory functions, improved behavior in navigating maze tests, and a reduction in plaque accumulation, suggesting TIM-3 therapy may be beneficial for Alzheimer’s in humans as well.
How many Alzheimer’s cases are linked to the TIM-3 molecule?
Approximately 90-95% of Alzheimer’s disease cases are late-onset, where the TIM-3 molecule has been identified as a genetic risk factor. Studies indicate that individuals with specific polymorphisms in the TIM-3 gene exhibit higher expression levels of this molecule, closely correlating with late-onset Alzheimer’s disease.
What are the potential next steps for TIM-3 therapy in human Alzheimer’s treatment?
Future steps for TIM-3 therapy in Alzheimer’s treatment include testing human anti-TIM-3 antibodies in mouse models that replicate Alzheimer’s pathology. Researchers aim to evaluate the therapy’s ability to halt plaque development and improve cognitive outcomes, paving the way for potential human clinical trials.
Are existing anti-TIM-3 antibodies suitable for Alzheimer’s disease treatment?
Yes, existing anti-TIM-3 antibodies, initially developed for cancer therapies, have the potential to be repurposed for treating Alzheimer’s disease. Their targeted action against the TIM-3 molecule may facilitate plaque clearance by reactivating microglia, thus providing a promising avenue for Alzheimer’s treatments.
What cognitive behaviors were improved in mice treated with TIM-3 therapy?
Mice treated with TIM-3 therapy demonstrated improved cognitive behaviors, particularly in memory recall and navigation tasks. Following plaque reduction, these mice exhibited increased ability to remember and exhibit survival-related behaviors, such as avoiding open spaces, indicating a restoration of cognitive functions.
What challenges exist in utilizing TIM-3 therapy for human Alzheimer’s patients?
Challenges in utilizing TIM-3 therapy for Alzheimer’s include ensuring that the therapy effectively crosses the blood-brain barrier and determining optimal dosing regimens that enhance microglial function without inducing excessive immune responses. Extensive clinical testing will be necessary to elucidate the therapy’s safety and efficacy.
What makes TIM-3 a promising target for Alzheimer’s therapy compared to traditional treatments?
TIM-3 is a promising target for Alzheimer’s therapy because it directly addresses the dysfunctional immune response in the brain, which traditional treatments often overlook. By focusing on activating microglia and enhancing plaque clearance, TIM-3 therapy may provide a more effective approach compared to existing anti-amyloid treatments that have shown limited efficacy.
| Key Point | Details |
|---|---|
| Study Focus | The research investigates the potential of TIM-3 therapy in treating Alzheimer’s disease by utilizing a strategy effective against certain cancers. |
| Mechanism of Action | By deleting TIM-3, the checkpoint molecule that inhibits microglia, the immune cells can effectively reduce amyloid plaques in the brain. |
| Importance of TIM-3 | TIM-3 is linked to late-onset Alzheimer’s and serves as an inhibitory signal preventing microglia from attacking harmful plaques. |
| Research Findings | In experiments with mice, deleting TIM-3 enhanced memory function and plaque clearance. |
| Therapeutic Application | Potential therapies could involve anti-TIM-3 antibodies or small molecules to inhibit TIM-3’s effects. |
| Next Steps | Ongoing research aims to assess the effectiveness of human anti-TIM-3 in Alzheimer’s mouse models. |
Summary
TIM-3 therapy for Alzheimer’s is emerging as a promising approach, leveraging insights from cancer treatment strategies. Recent findings indicate that targeting the TIM-3 immune checkpoint molecule can enhance the ability of microglia to clear amyloid plaques associated with Alzheimer’s disease, significantly improving memory and cognitive function in test subjects. This innovative strategy not only addresses the accumulation of harmful plaques in the brain but also opens up new avenues for treatment, marking a significant advancement in Alzheimer’s research.